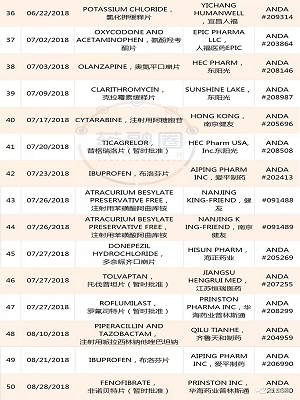
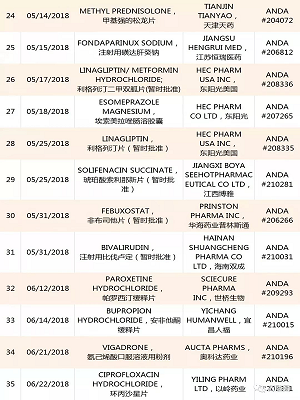
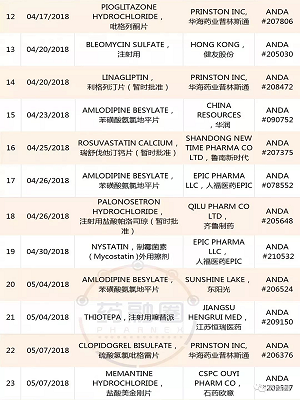
1. YILING Pharmaceutical:Letrozole ,ANDA #205869,20181114;
Anastrozole ,ANDA #206037,20181109;
2.AIPING Pharmaceutical:Benzonatate (苯佐那酯),ANDA #210562,20181109;
3.QILU Pharmaceutical齐鲁制药:Bortezomib ,ANDA #210824,20181109;
4.Hainan Puli:Ganciclovir ,ANDA #204204,20181108;
5.Huahai Pharmaceutical:Ticagrelor ,ANDA #208599,20181106;
6.ANBISON:Bupropion Hydrochloride ,ANDA #207224,20181105;
7.QILU Pharmaceutical Tadalafil ,ANDA #210420,20181104;
8.YAOPHARMA:Quinapril Hydrochloride ,ANDA #076803,20181102;
9.HAIZHNG Pharmaceutical:Irbesartan ,ANDA #206194,20181102;
10.Nantong Lianya:Metformin Hydrochloride,ANDA #209674,20181102;
More and more pharmaceutical enterprises, based on the domestic market, actively explore the US and European markets. The number of approvals has been accumulating, but the pharmaceutical companies, except Hengrui Pharmaceutical, Huahai Pharmaceutical, Nantong Lianya Pharmaceutical, Renfu Pharmaceutical and Qilu Pharmaceutical, have less export performance to the United States. According to the China Chamber of Commerce for Import and Export of Medical and Health Products, in the first quarter of 2018, China's exports of chemical preparations to the United States exceeded US$90 million, an increase of 18.8% over the same period last year. The 5 companies ranked the top in the export market, accounting for 67% of the total export volume.